
If you’ve ever fought off a cold, felt the nagging itch of a mosquito bite, or suffered from a scrape that promptly healed, you know that your immune system has been working properly to protect your health. Specially designed as our body’s defense from disease, our immune system safeguards us from any number of potentially harmful viruses, bacteria, and toxins each day by recognizing and reacting to foreign substances, broadly known as antigens.
For Leo Lefrançois, an immunology professor at the UConn Health Center and the immunology department’s new chairman, investigating the many unanswered questions about the inner workings of our immune response is likely to keep him busy for a lifetime.
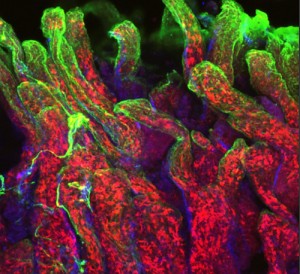
An important focus of his lab’s research efforts is in the field of mucosal immunology, and in particular on how the body responds to infection within the intestines. In addition, his lab is studying the effects of infection on other organs and trying to understand the basic underpinnings of immune system control.
Given these undertakings, it is perhaps no wonder that Lefrançois has numerous projects under way at any given time. With his mucosal immunology work, he is seeking to understand the nuances of immunity in a part of the body that is not only home to trillions of bacteria living in a symbiotic relationship with us, but that is also barraged with foreign material on a daily basis – that is, every time you eat anything.
“The gut can be considered the largest immune organ in the body because it’s so vast and covers so much surface area,” Lefrançois says. “But the immune response in the gut is also very complicated in terms of how it is orchestrated and regulated.”
Developing a Living Memory
Conducting research with certain types of bacteria and viruses, Lefrançois’ team is investigating how the immune response operates within various organs, including different parts of the intestine, after infection.
Of special interest to Lefrançois are T cells, a major player in the immune system. Many of the projects ongoing in Lefrançois’ lab are specifically aimed at understanding how T cell memory develops.
“When you get an infection or you get a vaccination, T cells respond to the infection,” Lefrançois says. “They get triggered to differentiate and grow. Their numbers increase dramatically, and then eventually most of those cells die off, and you end up with a small population of cells at the end – so-called memory cells – that will live for the lifetime of the organism.”
Along with antibodies, it is those memory cells that live on to protect you, should you be exposed to that particular antigen again later in life.
“That whole process – how it works – has really been a huge mystery in immunology for many, many years. It’s one of the Holy Grails that is still out there,” he says. “How do you identify those cells that are actually going to survive and become memory? Why do those few survive? Could we improve on that? Can we figure out how to manipulate that in some way?”
Another facet of his research, then, involves unraveling the details behind the very early immune response. How our immune system reacts within the first few hours or days after infection or vaccination, Lefrançois says, can affect how protective our immunological memory for that pathogen is going to be in the long term.
“Pieces of the puzzle have been discovered in the past few years from various labs around the world, including our own,” Lefrançois says. “But there are still a lot of gaps, and there probably will be well beyond my lifetime.”
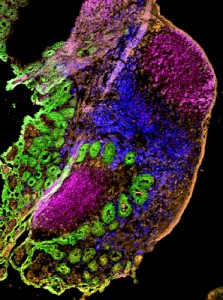
Visualizing the Fight Against Infection
Using imaging technology that has become available in recent years, it is also now possible for Lefrançois’ lab to see the actual anatomy of the immunological response. Special state-of-the-art microscopes allow Lefrançois’ team to compare many different pieces of tissue from distinct points in time, and equipment is now in place that even allows them to watch how the immune system responds to infection in real time.
“Using imaging, we’ve learned a lot about where the T cells are located during their activation after infection, how they’re moving,” he says. “It’s been incredibly powerful because you can see what’s happening as it happens. You can watch things move; you can measure their movement, their location, and with what they might be interacting.”
As new chair of the immunology department, Lefrançois says he is looking forward to recruiting additional faculty in the coming months. And though he may be spending less time in his lab nowadays, he is no less eager to witness its latest discoveries.
“I still get very excited about the work, and in the lab I have a great team whom I live through vicariously,” he says. “You always discover things you don’t expect – which, of course, is the fun part.”



