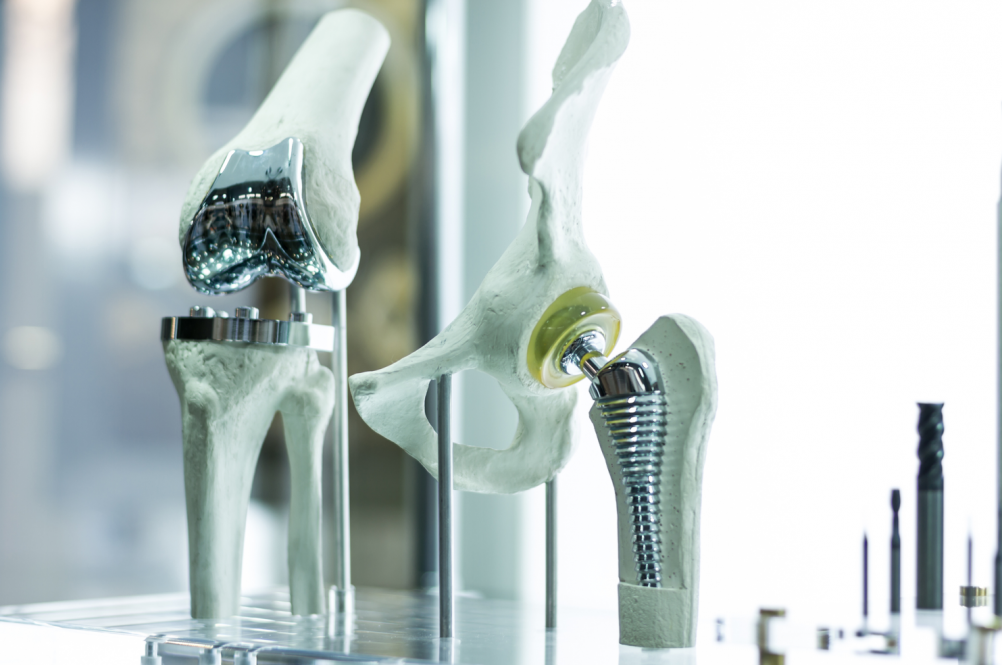
Cobalt was widely used for hip and knee joint replacements until cases of heavy metal poisoning appeared. Now, researchers from the University of Connecticut School of Medicine and Carnegie Mellon report a way to protect people with these implants from cobalt toxicity.
Globally, more than 2.9 million joint replacements are done every year, with the majority of them in the hip or knee. Cobalt-based alloys such as cobalt-chromium-molybdenum are widely used for these implants because of their strength and durability.
However, cobalt (and other metals) can be toxic if they accumulate in body tissues at high levels. And after several years of wear and tear on the implant, metal particles can build up in the area surrounding the joint, causing pain, inflammation, and dark discoloration. This type of localized heavy metal irritation is called metallosis. Even if the metal stays localized it can cause bone deterioration along with pain.
Removing the implants seems like the obvious solution, but revision joint surgery is invasive and has its risks. And traditional chelation therapy, in which a drug binds the heavy metal and allows the body to excrete it, has a difficult time penetrating into the joints and surrounding tissues.
Now, researchers in the Laurencin lab at UConn Health and Stephanie Sydlik at Carnegie Mellon report in the 30 October issue of PNAS that injecting a material made of a traditional chelator combined with a molecule naturally found in the fluid surrounding the joints makes an effective, less invasive therapy to clear out cobalt. The researchers used a chelator called British anti-Lewisite (BAL), a treatment originally invented to help soldiers poisoned with arsenic-containing Lewisite during World War II.
They attached the BAL to hyaluronic acid, a molecule commonly found in fluid that helps lubricate our joints. The BAL-hyaluronic acid was then injected into the hip joints of rats with cobalt metallosis. And within hours, the BAL-hyaluronic acid cleared a great deal of cobalt into the bloodstream and then the kidneys, so the rats could excrete it.
“This unique approached developed by teams at The Cato T. Laurencin Institute and the department of chemistry at Carnegie Mellon University offer a possible breakthrough treatment for metal ion disease after joint replacement,” says Dr. Cato Laurencin, the chief executive officer of the Cato T. Laurencin Institute for Regenerative Engineering, who is also both an orthopedic surgeon and a chemical engineer. “Now that we know it works in animals, our hope is to eventually bring this type of therapy to humans,” he says.