Doctors seeking a cure for an aggressive form of multiple sclerosis keep chasing a mirage: no matter how well a drug works in the lab, it never seems to help many patients in the clinic. But after closely examining stem cells from patients and their families, researchers think they know why the drugs coming out of labs are duds. Neuroscientist Dr. Stephen Crocker, associate professor in the Department of Neuroscience at UConn Health, and colleagues offer an explanation in the Feb. 1 issue of Experimental Neurology.
People with multiple sclerosis (MS) suffer from vision loss, pain, and paralysis that grows worse over time. The symptoms are from stray electrical signals in the nervous system. Our nerves operate like electrical wires, and just like wires, they need insulation around them. The insulation around our nerves is a substance called myelin, made by other cells in the brain called oligodendrocytes. For reasons that are not yet understood, the oligodendrocytes in people with multiple sclerosis don’t reliably repair the myelin. So people with MS gradually lose myelin, and their nerves start to short out like frayed wires, and eventually these neurons die.
[There] may be a kind of unique biology in each patient that determines their response. — Dr. L. John Greenfield
Most kinds of MS have a pattern of illness and then remission: symptoms flare up, then go away, then flare up again. There are effective drugs that help patients extend the periods of remission, and someone diagnosed with MS in his or her 20s may live comfortably for decades. But primary progressive MS (PPMS) is different. Patients diagnosed with PPMS get sicker steadily, without remission. And currently there are no drugs that reliably help.
Because PPMS patients have such an urgent need, many researchers look for possible treatments among medications that have already been approved for other illnesses. That way they can go right from lab to patient if they show promise. And so far many medications have shown promise – in the lab. But when they’re tested in actual PPMS patients, none of these drugs seems to do much.
Crocker and his colleagues at UConn Health wanted to know why. What was everyone missing in the search to treat PPMS?
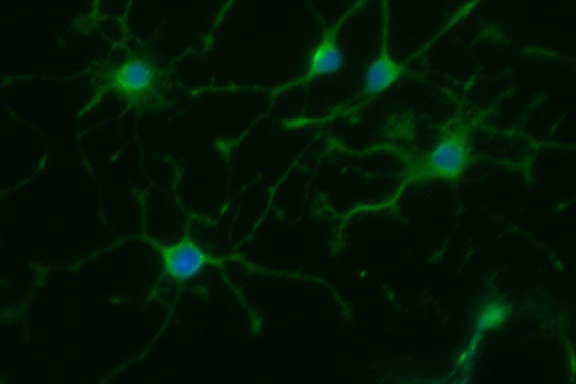
So the researchers collected blood cells from patients with PPMS, as well as the patients’ siblings, or spouses. Then in the lab, they “reprogrammed” them and turned them into neuroprogenitor stem cells. Stem cells can turn into any type of cell in the body; neuroprogenitor stem cells are found only in the brain, and specialize in turning into new brain and nerve tissue, such as the oligodendrocyte cells that re-myelinate nerves. These neuroprogenitor stem cells are known to protect the brain from injury, but this recent study was the first to ask whether these stem cells from someone with PPMS had the same ability to protect the brain as those from someone without the disease.
To explore this question, first the researchers tried adding the stem cells to brain tissues in animals with damage similar to PPMS. Stem cells from the healthy relatives and spouses started repairing the damaged areas. But the PPMS stem cells didn’t do anything.
“It’s like you bring in the National Guard to stem a riot, and [instead] they all sit down and start having lunch,” Crocker says.
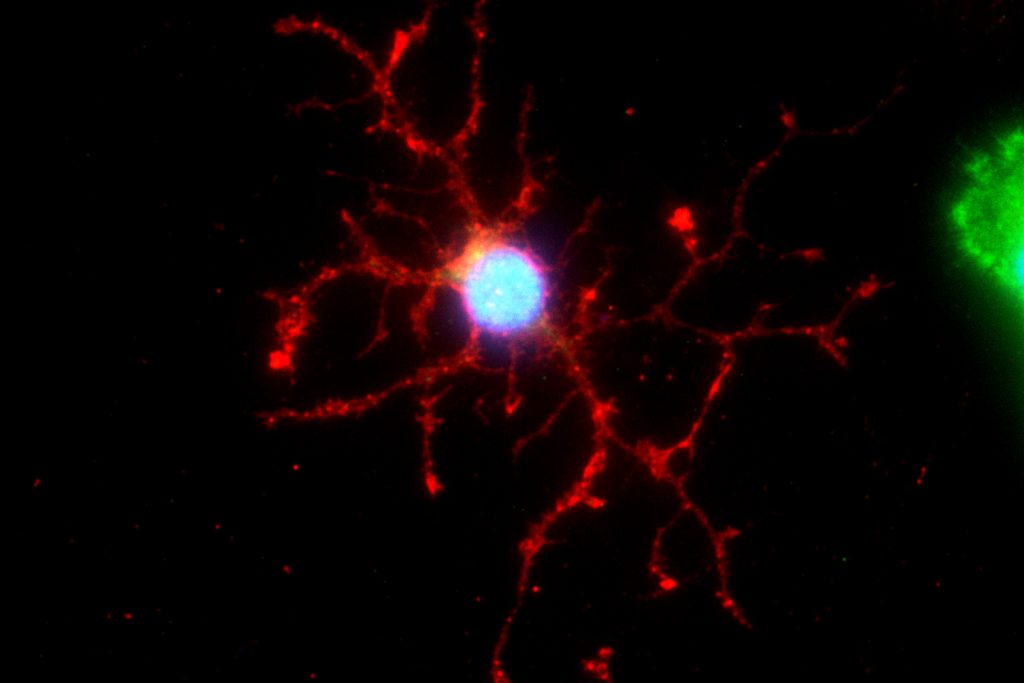
Dr. Crocker and his colleagues then tested how these stem cells were different by growing them in dishes in the lab. They collected the soup that the cells grew in, called ‘conditioning media,’ and tested how this affected other cells grown in it afterwards. The stem cells had left behind chemicals and proteins in the conditioned media, little messages that tell other cells that come later what they need to do. After conditioning the media with the stem cells, the researchers grew oligodendrocyte cells and measured how they matured in the dish. The cells grown in media conditioned by healthy stem cells matured into nice, big oligodendrocytes with no problems. But the cells added to dishes conditioned by PPMS stem cells didn’t mature at all. Something about the neural stem cells from PPMS patients was messing up the young oligodendrocytes, leading them astray.
So members of Crocker’s research team next tested some drug candidates for PPMS and added them to the young oligodendrocytes. The drugs absolutely helped the young oligodendrocytes mature when they were growing in media conditioned by stem cells from healthy people. But the same drugs didn’t help the young oligodendrocytes when they were grown in media conditioned by diseased stem cells. In those cases, the cells responded differently every time.
As Tolstoy might have said, healthy stem cells are all alike, but stem cells with primary progressive multiple sclerosis (PPMS) are all unhealthy in their own way. It might appear to be the same disease from the symptoms, but each patient’s PPMS seems to be caused by a different problem with that specific patient’s cells.
But that means that doctors may be able to screen drugs for brain repair on a patient-to-patient basis, Crocker says.
Dr. Matthew Tremblay, a neurologist who recently joined UConn Health and who treats patients with PPMS, has begun collaborating with Crocker as they plan the next steps to this research, looking to recruit patients for future studies. And in the lab they’ve already found that some drugs that have been dismissed as ineffective when tested using more typical techniques may have the potential to work very well for certain PPMS patients.
Crocker says the drugs never would have “risen above the noise” if cells from many different patients were tested as a group, instead of individually. Crocker, Tremblay, and their colleagues plan on continuing to test drug candidates on individual patients’ cells. They recently submitted several grant applications to continue and support this research.
And in addition to helping PPMS patients, this research shows that a patient’s own stem cells may not be useful as cure-alls. Many researchers are trying to develop therapies using a patient’s own stem cells, but that may be useless for diseases like PPMS that stem from problems with the stem cells themselves.
The research shows a novel way to use stem cells to find cures, says L. John Greenfield, chair of neurology at UConn Health. In situations like this, Greenfield says, there “may be a kind of unique biology in each patient that determines their response. That could mean this is a situation for precision medicine.”