UConn Health’s Neag Comprehensive Cancer Center is one of a few centers nationwide recruiting patients for a new clinical trial testing a novel immunotherapy’s ability to battle the most common and aggressive form of primary liver cancer called hepatocellular carcinoma (HCC).
At UConn Health the experimental intravenous immunotherapy medication OX40 of Pfizer (PF-04518600) is in the earliest phase of clinical trial testing for safety and efficacy. The immune stimulating drug fights cancer by binding to the OX40 ligand on immune system T Cells to help them rapidly multiply and live longer to hunt for and kill spreading cancer cells.
“Immunotherapy is the latest tool we are testing in the fight against advanced liver cancer,” says the clinical trial’s Principal Investigator Dr. Jeffrey Wasser, former medical director of the Clinical Trials Office at the Neag Comprehensive Cancer Center. “We hope our clinical trial testing of the immunotherapy OX40 will safely and effectively stimulate and boost the immune system of our liver cancer patients to fight back against their advanced disease.”
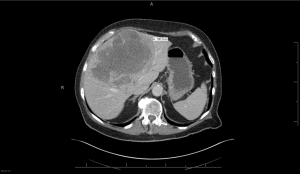
According to Wasser liver cancer is one of the most complicated diseases to stage and treat since it depends on a patient’s individual liver function. If liver cancer is caught early, it can be cured with surgery either by partial removal of the liver or a liver transplant. But if the majority of the liver is consumed by cancer or it has spread to other organs or surrounding blood vessels, surgery or a liver transplant are not possible.
This leaves a patient’s non-surgical liver cancer care options as chemotherapy, radiofrequency ablation, radiation beam therapy, radioembolization (placing tiny radioactive microbeads into the liver), or a transarterial chemoembolization (TACE) to block the blood supply fueling the cancer’s growth.
“If immunotherapy clinical trials prove effective, we hope these treatments will someday give non-transplant eligible liver cancer patients greater hope for a cure or long-term control of their cancer,” says Wasser.
The liver is located in the upper abdomen’s right side. Early liver cancer does not usually cause patients to experience symptoms. As the cancer advances some patients may feel pain in the upper abdomen, become full quickly when eating, have a swollen abdomen from fluid buildup, or notice a yellowing of their skin or eyes.
Most patients diagnosed with liver cancer already live with liver disease. Liver cancer in liver disease patients is often caught during their regular ultrasound monitoring. Patients at highest risk are those living with Hepatitis C, cirrhosis, chronic Hepatitis B infection, obesity or diabetes who may develop fatty liver.
UConn Health’s clinical trial site is currently recruiting patients with HCC primary liver cancer diagnoses who are not eligible for a cure with surgery or liver transplant since their disease has metastasized beyond the liver.
Wasser hopes to soon begin testing in clinical trials using OX40 in combination with another promising immune stimulating drug, Utolimumab (4 1-BB), for other cancers such as non-small cellular lung cancer that has progressed on prior immunotherapies and melanoma.
Interestingly, the research of Dr. Tony Vella and Dr. Adam Adler at UConn School of Medicine has led to the concept of the combined testing of OX40 with 4 1-BB after the research team’s mouse model studies showed an enhanced benefit.
This clinical trial research is sponsored by Pfizer, the maker of OX40 (PF-04518600) and Utomilumab.
To learn more about this clinical trial visit, here or the website of UConn Health’s Neag Comprehensive Cancer Center at: health.uconn.edu/cancer.