Mei Wei, an associate professor in the Department of Chemical, Materials and Biomolecular Engineering and the Institute of Materials Science, has recently received two large grants from the National Institutes of Health (NIH) and the National Science Foundation (NSF) that will allow her to expand upon her ongoing work in tissue regeneration and engineering.

She will work in collaboration with Dr. David Rowe, director of the Center for Regenerative Medicine and Skeletal Development and professor of Reconstructive Sciences in the School of Dental Medicine at the UConn Health Center.
For the NSF-funded study, she seeks to develop a scaffold that can mimic human tissue and encourage cartilage regeneration around joints. A project like this has important implications for joint disorders, especially osteoarthritis, a painful and debilitating disease.
The NIH-funded project involves the exploration of new bone imaging techniques that will offer researchers insight into the interaction of scaffolds and cells at different stages of bone repair.
Osteoarthritis is the top cause of chronic disability in the U.S., costing billions of dollars every year and incalculable pain for millions of people. Wei hopes her work will ultimately enable sufferers to undergo a procedure that would reverse the progression of osteoarthritis and reclaim their quality of life.
“Damage to the articular cartilage surface and the underlying bone can easily progress to joint degeneration, especially osteoarthritis,” says Wei.
To relieve the effects of osteoarthritis, the damage to the articular cartilage must be reversed. “Extensive efforts have been made in osteochondral [cartilage] defect treatment, but there is still no widely accepted method which produces consistent satisfactory results,” she says. All previous attempts at repairing or regenerating the cartilage have produced a replacement inferior to articular cartilage.
“The goal of this NSF project is to use a combination of a novel scaffold and an optimal cell source to effectively regenerate osteochondral cartilage with excellent functionality and long-term stability,” says Wei.

Biological scaffolds are artificial structures that can provide stability to regenerating tissue, making it easier for the cells to proliferate and develop. Wei is seeking to develop a graded scaffold that imitates the structure and properties of the articular cartilage and surrounding bone. The scaffold could be placed around a joint, providing temporary support. This would then be seeded with chondroprogenitor cells, which are cells that can develop into cartilage. As the cartilage develops, the support and protection to joints will be enhanced, mitigating the causes of osteoarthritis.
Wei’s imaging project focuses on developing means to watch the progress of bone repair procedures. As with cartilage regeneration, in bone repair a scaffold is seeded with the proper donor progenitor cells and placed at the site of the injury, facilitating regeneration.
To evaluate and analyze a certain repair technique, researchers would find it helpful to determine how the different components involved in repair, namely the scaffold and the different cells, are interacting. Current imaging platforms, however, do not allow real-time imaging of cell-cell or cell-scaffold interactions in living animals.
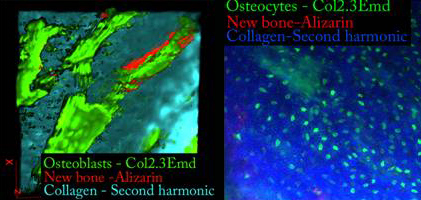
To overcome this problem, Wei and her team will be working with a transgenic mouse model to test a four-dimensional imaging technique that will be able to track the progression of different bone repair techniques. This technique takes advantage of the fact that every cell lineage shows a different color at different stages of development.
At the Health Center, Rowe will create transgenic mice in which a specific reporter protein is expressed when cells differentiate into a certain stage. These reporter proteins, called Green Fluorescent Proteins (GFP), give off a specific color when exposed to a specific wavelength of light.
With the GFP-labeled cells, Wei and her team can visualize cell-cell and cell-scaffold interactions and identify whether the cells originated from the original bone or from donor cells; how each of those sources of cells contributes to bone repair; and how those cells interact over time. This information can provide important insights into the analysis and development of new and existing bone repair procedures.
In addition to these grants, Wei has also recently received funding to organize a symposium at the Materials Research Society fall meeting, titled “Biomaterials for Tissue Regeneration.” This symposium will bring together 12 distinguished researchers in the field to present their work and facilitate the development of new, important research in the field of tissue regeneration.